A Viral Transport Medium (VTM) following the WHO and CDC recommendations with or without swab
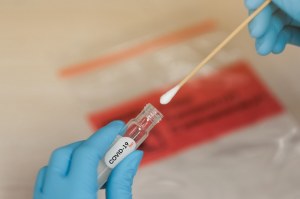
The success of the diagnosis of (2019-nCoV) during the C-19 outbreak depends largely on the quality of the specimen and the conditions under which the specimen is transported and stored before being processed in the laboratory.
Commercially prepared viral transport media are available in a screw cap plastic tube containing buffered proteins (serum, albumin or gelatin) and antibiotics. Antibiotics are usually incorporated into the viral transport media to suppress the growth of contaminating bacteria and fungi, so separate samples from the same site should be collected if bacterial or fungal cultures are also requested.
The product is provided as a liquid in a sterile 13 ml flat bottom tube with or without a swab to provide a maximum range of possibilities to collect samples.
The VTM is available in 2 formats : Without and with swabs.
Description: Viral Transport Medium (VTM) Viral Transport Medium (VTM) with swabs
Size: 49 vials of 3 ml 49 vials of 3 ml + 49 swabs
Medical devices for in vitro diagnosis. Read the operating instructions carefully.
